VRPX Stock: Key Takeaways
- Virpax Pharmaceuticals completes the first step with the FDA toward approval of its product to prevent transmission of viruses like COVID-19 and influenza.
- Animal studies have shown success with no adverse effects reported.
- VRPX stock surges more than 280% as the company says it’s preparing for human clinical trials.
Virpax Pharmaceuticals, Inc. (NASDAQ: VRPX) more than tripled today on FDA-related news about its anti-viral barrier product.
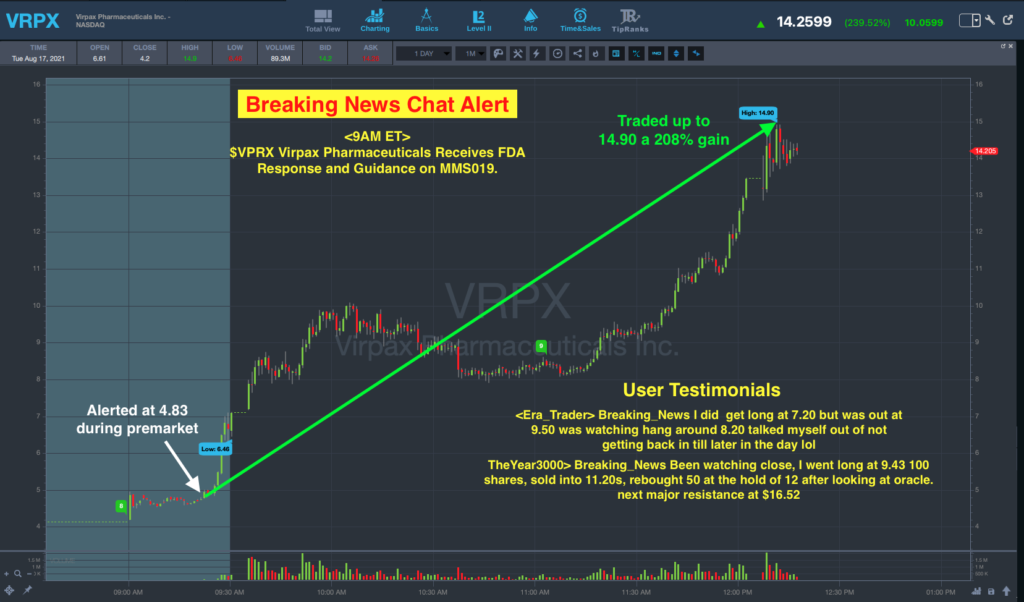
Trading of VRPX stock was halted repeatedly in today’s session, and at writing, shares were up more than 286%.
The rally comes after Virpax announced it received a written pre-investigational new drug (pre-IND) response from the FDA for MMS019.
Blocking Transmission of Viruses Like COVID
MMS019 is Virpax’s “patented and proprietary high-density molecular masking spray under development for use as an anti-viral barrier product” to prevent transmission of viruses like COVID-19 and influenza.
It’s a nasal spray type of treatment “based on a type of nanotechnology that enables the exclusive delivery of a metabolically labile peptide drug into the brain.”
Previous in-vitro, ex-vivo (human mucosal cells), and in-vivo trials for MMS019 “demonstrated inhibition of viral replication of SARS-CoV-2 and influenza in animals at much higher ranges than what is encountered by humans in the nasal passages.”
The company says no adverse effects were observed during those studies.

Image credit: Josep Suria/Shutterstock.com
The animal studies also “demonstrated inhibition of viral replication of SARS-CoV-2 and influenza in animals at much higher ranges than what is encountered by humans in the nasal passages.”
Virpax said it “believes the results of the pre-IND response support further research on MMS019 as an intranasal protective that may limit transmission of the viruses to others.”
Now Virpax plans to pursue a New Drug Application (NDA) to approve MMS019 as a daily intranasal treatment.
The FDA said that approval can come from the Office of Non-Prescription Drugs and Virpax is working with Syneos Health to assist with the optimal clinical trial design for humans.
The FDA’s pre-IND guidance helps ensure a drug’s clinical trials and studies meet the agency’s requirements for future approval.
Virpax’s chairman and CEO Anthony P. Mack said, “We are very pleased with the response from the FDA. We believe that the initial pathway to move forward with the development of MMS019 has been clarified. The pre-IND meeting provides an opportunity for open communication between the Sponsor and the FDA to discuss the IND development plan and to obtain the FDA’s guidance for clinical studies for the new drug candidate.”
Mack said the company plans to seek approval for the drug as an over-the-counter treatment rather than a prescription drug.
STT Breaking News Team Notices VRPX Stock
The StocksToTrade Breaking News Team alerted on this stock ahead of the market open today.
At 9:00 a.m. Eastern, an alert was sent which read, “$VRPX Virpax Pharmaceuticals Receives FDA Response and Guidance on MMS019.”
At the time of that alert, the stock was at $4.83 per share in premarket trade.
VRPX then opened at $6.61 per share was off to the races throughout the session.
Eight Facts About VRPX Stock
- Virpax Pharmaceuticals was founded in 2016 and is headquartered in West Chester, PA.
- The company specializes in developing non-addictive pain treatments to “target a patient’s pain at its source”.
- Virpax is developing novel drug-delivery systems and drug-releasing technologies.
- The company has global rights to a proprietary patented Molecular Envelope Technology (MET).
- MET uses an intranasal device to deliver enkephalin via nanoparticles for the management of acute and chronic pain.
- Virpax also has global rights to a patented metered-dose topical spray film delivery technology for musculoskeletal pain and a patented injectable “local anesthetic” liposomal hydrogel technology for postoperative pain management.
- VRPX stock closed at $4.20 per share on Monday.
- The stock opened at $6.61 per share today and blew past $16 per share by 1:25 p.m. Eastern.
Featured cover image credit: ranjith ravindran/Shutterstock.com