- Therapeutic Solutions receives FDA clearance for next step in registering its COVID-19 stem cell therapy…
- Company believes treatment is vital for unvaccinated and breakthrough COVID infections…
- STT Breaking News Chat nails alert before the open…
Therapeutic Solutions International Inc (OTCMKTS: TSOI) more than doubled today on news about its COVID-19 therapy.
TSOI stock popped as much as 112% early in the session and was trading 50% higher at writing.
The rally comes after Therapeutic Solutions announced it received “clearance from the Food and Drug Administration (FDA) to initiate a Phase III pivotal trial for registration of the Company’s JadiCell™ universal donor stem cell as a treatment for COVID-19 associated lung failure.”
If you’re a subscriber to the STT Breaking News Chat, this stock might look familiar.
STT Breaking News Team Alerts TSOI Stock
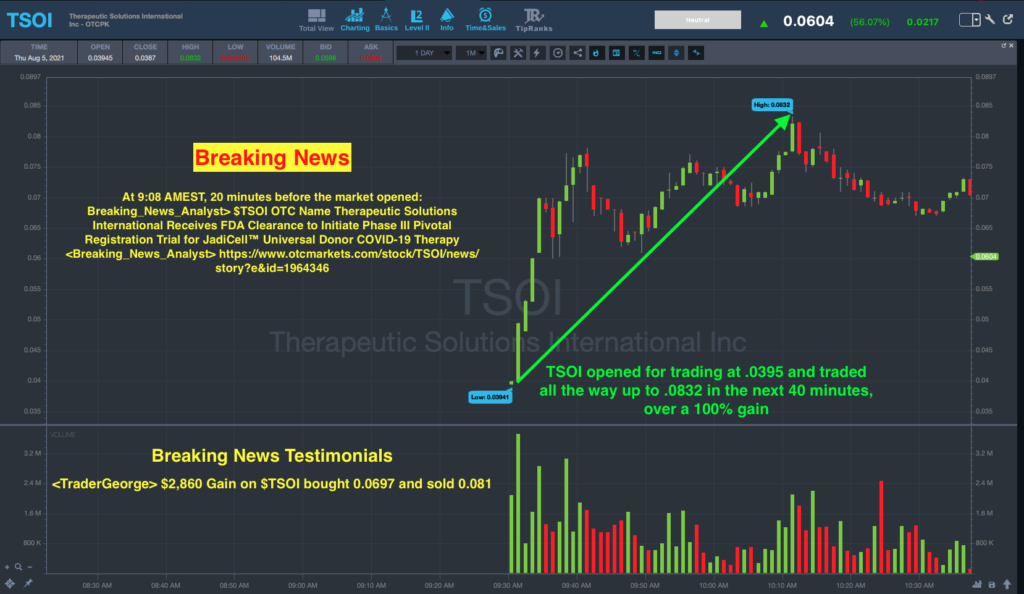
The Breaking News Team was all over this news in the morning, sending out their first alert on TSOI stock 20 minutes before the open.
The alert was sent out at 9:08 a.m. Eastern reading:
“$TSOI OTC Name Therapeutic Solutions International Receives FDA Clearance to Initiate Phase III Pivotal Registration Trial for JadiCell™ Universal Donor COVID019 Therapy”.
A look at the chart shows TSOI stock opened the day at $0.0395 and then surged to a daily high of $0.0832 in the next 40 minutes.
That was a gain of 112% by 10:11 a.m. Eastern.
One testimonial from the chat said, “$2,860 Gain on $TSOI bought 0.0697 and sold 0.081”.
More About JadiCell
Therapeutic Solutions said previous studies have “demonstrated the superior activity of JadiCell™ to other types of stem cells including bone marrow, adipose, cord blood, and placenta.”
For COVID-19 treatment, the company conducted a double-blind placebo-controlled clinical trial with patients in the ICU on a ventilator.
That trial showed the treatment was 100% effective in saving the lives of patients under 85 and 91% effective for those over 85.
The next step is the Phase III pivotal trial to be able to register the treatment with the FDA to be used.
Dr. Thomas E. Ichim, Director of Therapeutic Solutions, said, “FDA clearance to initiate a Phase III clinical trial means we are at the last phase of development before commercially selling the product. This positions us in a highly exclusive place in that to our knowledge no other cells have this potent ability to concurrently suppress inflammation while restoring function of tissue damaged by SAR-CoV-2.”
“Having personally seen the effects of JadiCells on patients, I have seen their clinical potential firsthand” said Dr. James Veltmeyer, Chief Medical Officer of the Company. “I am very excited to enter the final step of clinical development before being able to provide these cells to the general population.”
Therapeutic Solutions believes their treatment is especially vital at this time as more Americans seem to be resistant to getting the COVID-19 vaccine.
“Despite the initial promise of vaccine approaches, there exists a significant portion of the population refusing them and there are also patients in whom vaccines have failed to induce appropriate immunity. Once COVID-19 initiates its pathological cascade leading to lung failure, no therapies exist until now to address this population” said Famela Ramos, Vice President of Business Development.
Timothy Dixon, President and CEO of Therapeutic Solutions International, said, “Successful completion of the agreed-upon trial with the FDA will position the Company as a significant force in the global battle against this unseen enemy that to date has caused over 4.25 million deaths. We are extremely proud of our progress and vow to accelerate our work for humanity and for our shareholders.”
Five Things to Know About TSOI Stock
- Therapeutic Solutions International was founded in 2011 and is headquartered in Oceanside, CA.
- The company is “focused on immune modulation for the treatment of several specific diseases.”
- Its product line includes Nutraceuticals, StemVacs, and the JadiCell treatment.
- Trading volume surged to nearly 2,500% average.
- The company has an $87 million market cap.
Featured cover image credit: ranjith ravindran/Shutterstock.com